Ask any Matric student, and they will tell you: “If you survive Grade 11 Physics, Matric is easy.”
Grade 11 is the year where the “training wheels” come off. In Physics, you stop working in one dimension (left/right) and start working in two dimensions (angles and slopes). In Chemistry, you move from simply balancing equations to explaining the invisible forces that hold the universe together.
This guide breaks down the curriculum into the strategies you need to master to get that distinction.
Paper 1: Physics (The Mechanics of the Universe)
Focus: Newton’s Laws, Electrostatics, and Electromagnetism.
1. Vectors in 2D (The New Skill)
This is the single most important skill you will learn this year. Almost every question in Paper 1—from Newton’s Laws to Electrostatics—relies on your ability to resolve vectors.
- The Triangle Rule: You must be comfortable taking a force at an angle and splitting it into its x (horizontal) and y (vertical) components using SOH CAH TOA.
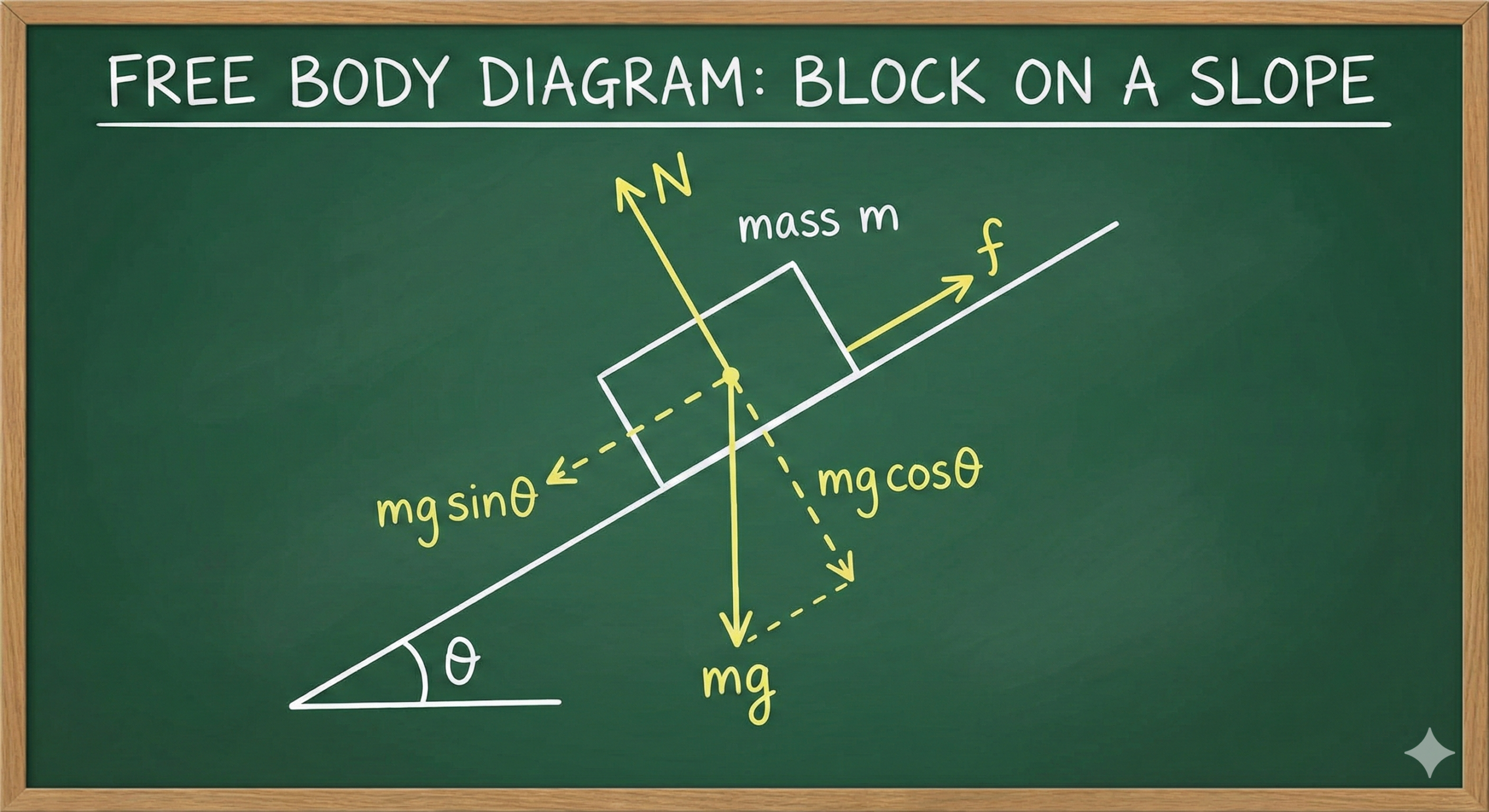
- The Slope Trap: When an object is on an inclined plane, Gravity (F_g) acts straight down, but the motion is parallel to the slope. You must learn to resolve Gravity into:
- F_{g\parallel} = mg \sin\theta (Slides it down the slope).
- F_{g\perp} = mg \cos\theta (Presses it into the slope).
2. Newton’s Laws
In Grade 11, Newton’s Second Law (F_{net} = ma) becomes your religion.
- Connected Bodies: You will be asked to calculate the tension in a rope connecting two blocks.
- Strategy: Treat the two blocks as one system first to find the acceleration. Then, isolate one block to find the tension.
- Friction: Remember that Friction (f_k) always acts opposite to the direction of motion, not necessarily the direction of the force.
3. Electrostatics (Coulomb’s Law)
We move from “rubbing balloons” to calculating the exact force between charges.
- The Inverse Square Law: Formula:
F = k \frac{Q_1 Q_2}{r^2}
- Distinction Tip: Pay attention to r^2. If you double the distance, the force becomes four times weaker (not two times).
- 2D Problems: Just like with forces, you will have to calculate the Net Electric Field at a point caused by three different charges arranged in a triangle. This brings you right back to Vectors.
Paper 2: Chemistry (Matter and Change)
Focus: Chemical Bonding, IMFs, and Stoichiometry.
1. Intermolecular Forces (IMFs)
This is the “make or break” section of Paper 2. It connects the microscopic structure to macroscopic properties (Boiling Point, Melting Point).
- The Hierarchy: You must memorize the strength order:
- Hydrogen Bonds: (Strongest) Occurs in H−O, H−N, H−F.
- Dipole-Dipole: (Medium) Occurs between polar molecules.
- London Forces: (Weakest) Occurs between non-polar molecules.
- The “Explanation” Recipe: When asked “Why does water have a higher boiling point than Ammonia?”, use this 4-step structure:
- Identify the type of IMF in both.
- Compare the strength of the forces.
- State that more energy is needed to overcome the stronger forces.
- Therefore, the boiling point is higher.
2. Stoichiometry (The Mole)
This is the mathematics of Chemistry. In Grade 11, you deal with Limiting Reagents.
- The Concept: If you are baking cakes and you have 50 eggs but only 1 cup of flour, the flour dictates how many cakes you can make. It is the “Limiting Reagent.”
- The Method: Always calculate the number of moles (n) for both reactants. Compare the ratio to the balanced equation to see which one runs out first.
3. Gas Laws
You need to master the Ideal Gas Law: pV = nRT.
- The Trap: Units!
- Pressure (p) must be in Pascals (Pa), not kPa.
- Volume (V) must be in m^3, not dm^3 or cm^3.
- Temperature (T) must be in Kelvin (^\circ C + 273).
- If you forget to convert your units, your answer will be wildly wrong.
Decksh’s Top 3 Tips for a Distinction
Tip 1: Master the “Free Body Diagram”
In Physics, never start a calculation without drawing a Free Body Diagram (FBD).
- It clears your mind.
- It earns you marks (usually 2-3 marks just for the drawing).
- Rule: Draw the dot (the object) and arrows pointing away from the dot. Label every arrow (F_g, F_N, F_{app}, f_k).
Tip 2: Definitions are “Free Marks”
Physics and Chemistry exams define terms very specifically.
- You cannot define “Activation Energy” in your own words. You must say: “The minimum energy required for a reaction to take place.”
- The Department of Education provides a list of definitions. Memorize them word-for-word.
Tip 3: Don’t ignore the “Graph” questions
In Chemistry (Reaction Rates) and Physics (Motion), graphs tell a story.
- Gradient: What does the slope represent? (e.g., In a Velocity-Time graph, the gradient is Acceleration).
- Area: What does the area under the graph represent? (e.g., In a Velocity-Time graph, the area is Displacement).
Conclusion
Grade 11 Physical Sciences is about building a toolkit. You are learning the tools (Vectors, Moles, IMFs) that you will use to solve complex problems in Matric. Don’t just memorize the formulas; understand what they mean. If you can resolve a vector on a slope and explain why ice floats, you are ready for a distinction.
Good luck!
